Autism spectrum disorder
Autism spectrum disorder is a construct used to describe individuals with a specific combination of impairments in social communication and repetitive behaviours, highly restricted interests and/or sensory behaviours beginning early in life. The worldwide prevalence of autism is just under 1%, but estimates are higher in high-income countries. Although gross brain pathology is not characteristic of autism, subtle anatomical and functional differences have been observed in post-mortem, neuroimaging and electrophysiological studies. Initially, it was hoped that accurate measurement of behavioural phenotypes would lead to specific genetic subtypes, but genetic findings have mainly applied to heterogeneous groups that are not specific to autism. Psychosocial interventions in children can improve specific behaviours, such as joint attention, language and social engagement, that may affect further development and could reduce symptom severity. However, further research is necessary to identify the long-term needs of people with autism, and treatments and the mechanisms behind them that could result in improved independence and quality of life over time. Families are often the major source of support for people with autism throughout much of life and need to be considered, along with the perspectives of autistic individuals, in both research and practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
133,45 € per year
only 133,45 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others

Neurogenetic disorders across the lifespan: from aberrant development to degeneration
Article 05 January 2022
Neuroimaging genetics approaches to identify new biomarkers for the early diagnosis of autism spectrum disorder
Article Open access 17 April 2023
Symptoms of autism in Williams syndrome: a transdiagnostic approach
Article Open access 30 July 2024
References
- Lord, C. et al. Autism from 2 to 9 years of age. Arch. Gen. Psychiatry63, 694–701 (2006). This paper establishes that autism is a stable diagnosis (as a spectrum) beginning at least by 2 years of age. The paper also establishes parent interview and clinician observation as predictive of autism at 9 years of age. Finally, it is the first paper that shows that the specific DSM-IV-TR diagnoses is unstable across childhood but that the instability is almost all shifting across categories not outside the spectrum.ArticlePubMedGoogle Scholar
- Risi, S. et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry45, 1094–1103 (2006). ArticlePubMedGoogle Scholar
- Loomes, R., Hull, L. & Mandy, W. P. L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry56, 466–474 (2017). ArticlePubMedGoogle Scholar
- Brugha, T. S. et al. Epidemiology of autism in adults across age groups and ability levels. Br. J. Psychiatry209, 498–503 (2016). This paper uses active case-finding to provide representative estimates of the prevalence of autism and demonstrated that rates of autism in men and women are equivalent in adults with moderate-to-profound intellectual disability.ArticlePubMedGoogle Scholar
- Brugha, T., Bankart, J., McManus, S. & Gullon-Scott, F. CDC autism rate: misplaced reliance on passive sampling? Lancet392, 732–733 (2018). ArticlePubMedGoogle Scholar
- Baxter, A. J. et al. The epidemiology and global burden of autism spectrum disorders. Psychol. Med.45, 601–613 (2015). ArticleCASPubMedGoogle Scholar
- Elsabbagh, M. et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res.5, 160–179 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Magnusson, C. et al. Migration and autism spectrum disorder: population-based study. Br. J. Psychiatry201, 109–115 (2012). ArticlePubMedGoogle Scholar
- Goodman, R. & Richards, H. Child and adolescent psychiatric presentations of second-generation Afro-Caribbeans in Britain. Br. J. Psychiatry167, 362–369 (1995). ArticleCASPubMedGoogle Scholar
- Dyches, T. T., Wilder, L. K., Sudweeks, R. R., Obiakor, F. E. & Algozzine, B. Multicultural issues in autism. J. Autism Dev. Disord.34, 211–222 (2004). ArticlePubMedGoogle Scholar
- Keen, D. V., Reid, F. D. & Arnone, D. Autism, ethnicity and maternal immigration. Br. J. Psychiatry196, 274–281 (2010). ArticleCASPubMedGoogle Scholar
- McManus, S., Bebbington, P., Jenkins, R. & Brugha, T. Adult Psychiatric Morbidity Survey: mental health and wellbeing in England, 2014. NHShttps://digital.nhs.uk/data-and-information/publications/statistical/adult-psychiatric-morbidity-survey/adult-psychiatric-morbidity-survey-survey-of-mental-health-and-wellbeing-england-2014 (2016).
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet392, 1789–1858 (2018). ArticleGoogle Scholar
- Marcheselli, F. et al. Mental health of children and young people in England, 2017. NHShttps://digital.nhs.uk/data-and-information/publications/statistical/mental-health-of-children-and-young-people-in-england/2017/2017 (2018).
- Brugha, T. C. et al. Autism Spectrum Disorder, Adult Psychiatric Morbidity Survey 2014. (2014).
- Lundstrom, S., Reichenberg, A., Anckarsater, H., Lichtenstein, P. & Gillberg, C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ350, h1961 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Tromans, S., Chester, V., Kiani, R., Alexander, R. & Brugha, T. The prevalence of autism spectrum disorders in adult psychiatric inpatients: a systematic review. Clin. Pract. Epidemiol. Ment. Health14, 177–187 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Modabbernia, A., Velthorst, E. & Reichenberg, A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol. Autism8, 13 (2017). ArticlePubMedPubMed CentralCASGoogle Scholar
- Wu, S. et al. Advanced parental age and autism risk in children: a systematic review and meta-analysis. Acta Psychiatr. Scand.135, 29–41 (2017). ArticleCASPubMedGoogle Scholar
- Taylor, L. E., Swerdfeger, A. L. & Eslick, G. D. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine32, 3623–3629 (2014). ArticleCASPubMedGoogle Scholar
- Lai, M.-C., Lombardo, M. V. & Baron-Cohen, S. Autism. Lancet383, 896–910 (2014). ArticlePubMedGoogle Scholar
- Velikonja, T., Fett, A.-K. & Velthorst, E. Patterns of nonsocial and social cognitive functioning in adults with autism spectrum disorder: a systematic review and meta-analysis. JAMA Psychiatry76, 135–151 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- McNally Keehn, R. H., Lincoln, A. J., Brown, M. Z. & Chavira, D. A. The coping cat program for children with anxiety and autism spectrum disorder: a pilot randomized controlled trial. J. Autism Dev. Disord.43, 57–67 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Jones, E. J. H., Gliga, T., Bedford, R., Charman, T. & Johnson, M. H. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci. Biobehav. Rev.39, 1–33 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Ozonoff, S. et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics128, e488–e495 (2011). PubMedPubMed CentralGoogle Scholar
- Jones, R. M. & Lord, C. Diagnosing autism in neurobiological research studies. Behav. Brain Res.251, 113–124 (2013). ArticlePubMedGoogle Scholar
- Johnson, M. H. Autism: demise of the innate social orienting hypothesis. Curr. Biol.24, R30–R31 (2014). ArticleCASPubMedGoogle Scholar
- Johnson, M. H., Jones, E. J. H. & Gliga, T. Brain adaptation and alternative developmental trajectories. Dev. Psychopathol.27, 425–442 (2015). ArticlePubMedGoogle Scholar
- The Lancet Psychiatry. Of mice and mental health. Lancet Psychiatry6, 877 (2019). ArticleCASPubMedGoogle Scholar
- Nelson, C. A. et al. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev. Psychopathol.14, 499–520 (2002). ArticlePubMedGoogle Scholar
- Cross-Disorder Group of the Psychiatric Genomics Consortium et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet.45, 984–994 (2013). ArticleCASGoogle Scholar
- Gaugler, T. et al. Most genetic risk for autism resides with common variation. Nat. Genet.46, 881–885 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, K., Gaitsch, H., Poon, H., Cox, N. J. & Rzhetsky, A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet.49, 1319–1325 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sanders, S. J. et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron87, 1215–1233 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Satterstrom, F. K. et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Preprint at https://doi.org/10.1101/484113 (2019).
- Sanders, S. J. et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature485, 237–241 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Neale, B. M. et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature485, 242–245 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- O’Roak, B. J. et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature485, 246–250 (2012). ArticlePubMedPubMed CentralCASGoogle Scholar
- Sanders, S. J. et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron70, 863–885 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Levy, D. et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron70, 886–897 (2011). ArticleCASPubMedGoogle Scholar
- Sebat, J. et al. Strong association of de novo copy number mutations with autism. Science316, 445–449 (2007). This paper is the first to focus explicitly on simplex autism and show the importance of de novo CNVs in simplex cases, versus familial cases, versus controls.ArticleCASPubMedPubMed CentralGoogle Scholar
- Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet.51, 431–444 (2019). ArticleCASPubMedPubMed CentralGoogle Scholar
- Willsey, J. et al. De novo coding variants are strongly associated with Tourette syndrome. Eur. Neuropsychopharmacol.29, S737 (2019). ArticleGoogle Scholar
- Epi4K Consortium. Epi4K: gene discovery in 4,000 genomes. Epilepsia53, 1457–1467 (2012). ArticleGoogle Scholar
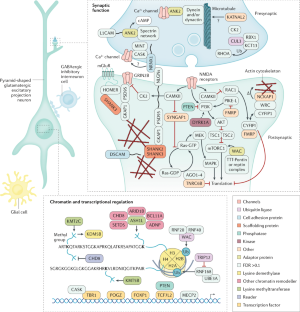
- Jamain, S. et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet.34, 27–29 (2003). This is the first paper to show a de novo loss-of-function mutation in a synaptic gene associated with non-syndromic autism and was a harbinger for many of the findings that came after.ArticleCASPubMedPubMed CentralGoogle Scholar
- Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature515, 216–221 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature515, 209–215 (2014). ArticlePubMedPubMed CentralCASGoogle Scholar
- Sestan, N. & State, M. W. Lost in translation: traversing the complex path from genomics to therapeutics in autism spectrum disorder. Neuron100, 406–423 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- State, M. W. & Sestan, N. The emerging biology of autism spectrum disorders. Science337, 1301–1303 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature511, 421–427 (2014). ArticlePubMed CentralCASGoogle Scholar
- Devlin, B. & Scherer, S. W. Genetic architecture in autism spectrum disorder. Curr. Opin. Genet. Dev.22, 229–237 (2012). ArticleCASPubMedGoogle Scholar
- de la Torre-Ubieta, L., Won, H., Stein, J. L. & Geschwind, D. H. Advancing the understanding of autism disease mechanisms through genetics. Nat. Med.22, 345–361 (2016). ArticlePubMedPubMed CentralCASGoogle Scholar
- SFARI Gene Website. https://gene.sfari.org/ (2019).
- Parikshak, N. N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell155, 1008–1021 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ben-David, E. & Shifman, S. Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol. Psychiatry18, 1054–1056 (2013). ArticleCASPubMedGoogle Scholar
- Willsey, A. J. et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell155, 997–1007 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Pinto, D. et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature466, 368–372 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Gilman, S. R. et al. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron70, 898–907 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Fuccillo, M. V. Striatal circuits as a common node for autism pathophysiology. Front. Neurosci.10, 27 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Velmeshev, D. et al. Single-cell genomics identifies cell type-specific molecular changes in autism. Science364, 685–689 (2019). ArticleCASPubMedPubMed CentralGoogle Scholar
- Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med.377, 1713–1722 (2017). ArticleCASPubMedGoogle Scholar
- Mercuri, E. et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med.378, 625–635 (2018). ArticleCASPubMedGoogle Scholar
- Matharu, N. et al. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science363, eaau0629 (2019). ArticleCASPubMedGoogle Scholar
- Abudayyeh, O. O. et al. RNA targeting with CRISPR–cas13. Nature550, 280–284 (2017). ArticlePubMedPubMed CentralCASGoogle Scholar
- Power, J. D. et al. Customized head molds reduce motion during resting state fMRI scans. NeuroImage189, 141–149 (2019). ArticlePubMedGoogle Scholar
- Solso, S. et al. Diffusion tensor imaging provides evidence of possible axonal overconnectivity in frontal lobes in autism spectrum disorder toddlers. Biol. Psychiatry79, 676–684 (2016). ArticlePubMedGoogle Scholar
- Clements, C. C. et al. Evaluation of the social motivation hypothesis of autism: a systematic review and meta-analysis. JAMA Psychiatry75, 797–808 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Ecker, C. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch. Gen. Psychiatry69, 195–209 (2012). ArticlePubMedGoogle Scholar
- Langen, M. et al. Changes in the development of striatum are involved in repetitive behavior in autism. Biol. Psychiatry76, 405–411 (2014). ArticlePubMedGoogle Scholar
- Elsabbagh, M. & Johnson, M. H. Autism and the social brain: the first-year puzzle. Biol. Psychiatry80, 94–99 (2016). ArticlePubMedGoogle Scholar
- Courchesne, E. et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology57, 245–254 (2001). ArticleCASPubMedGoogle Scholar
- Hazlett, H. C. et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch. Gen. Psychiatry62, 1366–1376 (2005). ArticlePubMedGoogle Scholar
- Wolff, J. J. et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry169, 589–600 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Hazlett, H. C. et al. Early brain development in infants at high risk for autism spectrum disorder. Nature542, 348–351 (2017). This seminal paper, through careful recruitment and methodology, was the first to show significant early differences that may contribute to our understanding of developmental features in neural structure and circuits. ArticleCASPubMedPubMed CentralGoogle Scholar
- Wolff, J. J. et al. Neural circuitry at age 6 months associated with later repetitive behavior and sensory responsiveness in autism. Mol. Autism8, 8 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Emerson, R. W. et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci. Transl. Med.9, eaag2882 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Smith, E. et al. Cortical thickness change in autism during early childhood: CT in early childhood ASD. Hum. Brain Mapp.37, 2616–2629 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Uddin, L. Q., Dajani, D. R., Voorhies, W., Bednarz, H. & Kana, R. K. Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl. Psychiatry7, e1218 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Herringshaw, A. J., Ammons, C. J., DeRamus, T. P. & Kana, R. K. Hemispheric differences in language processing in autism spectrum disorders: a meta-analysis of neuroimaging studies. Autism Res.9, 1046–1057 (2016). ArticlePubMedGoogle Scholar
- He, Y., Byrge, L. & Kennedy, D. P. Non-replication of functional connectivity differences in autism spectrum disorder across multiple sites and denoising strategies. Preprint at https://doi.org/10.1101/640797 (2019).
- Lawrence, K. E., Hernandez, L. M., Bookheimer, S. Y. & Dapretto, M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res.12, 53–65 (2019). ArticlePubMedGoogle Scholar
- Plitt, M., Barnes, K. A., Wallace, G. L., Kenworthy, L. & Martin, A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc. Natl Acad. Sci. USA112, E6699–E6706 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Di Martino, A. et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry19, 659–667 (2014). ArticlePubMedGoogle Scholar
- Doyle-Thomas, K. A. R. et al. Atypical functional brain connectivity during rest in autism spectrum disorders. Ann. Neurol.77, 866–876 (2015). ArticlePubMedGoogle Scholar
- Supekar, K. et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep.5, 738–747 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dajani, D. R. & Uddin, L. Q. Local brain connectivity across development in autism spectrum disorder: a cross-sectional investigation. Autism Res.9, 43–54 (2016). ArticlePubMedGoogle Scholar
- Hull, J. V. et al. Resting-state functional connectivity in autism spectrum disorders: a review. Front. Psychiatry7, 205 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Lombardo, M. V. et al. Different functional neural substrates for good and poor language outcome in autism. Neuron86, 567–577 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Carlisi, C. O. et al. Disorder-specific and shared brain abnormalities during vigilance in autism and obsessive-compulsive disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging2, 644–654 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Alaerts, K., Swinnen, S. P. & Wenderoth, N. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci.11, 1002–1016 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Kirkovski, M., Enticott, P. G., Hughes, M. E., Rossell, S. L. & Fitzgerald, P. B. Atypical neural activity in males but not females with autism spectrum disorder. J. Autism Dev. Disord.46, 954–963 (2016). ArticlePubMedGoogle Scholar
- Venkataraman, A. et al. Pivotal response treatment prompts a functional rewiring of the brain among individuals with autism spectrum disorder. NeuroReport27, 1081–1085 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Levisohn, P. M. The autism-epilepsy connection. Epilepsia48, 33–35 (2007). ArticlePubMedGoogle Scholar
- Cantor, D. S., Thatcher, R. W., Hrybyk, M. & Kaye, H. Computerized EEG analyses of autistic children. J. Autism Dev. Disord.16, 169–187 (1986). ArticleCASPubMedGoogle Scholar
- Lefebvre, A. et al. Alpha waves as a neuromarker of autism spectrum disorder: the challenge of reproducibility and heterogeneity. Front. Neurosci.12, 662 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Tierney, A. L., Gabard-Durnam, L., Vogel-Farley, V., Tager-Flusberg, H. & Nelson, C. A. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLOS ONE7, e39127 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Oberman, L. M. et al. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res.24, 190–198 (2005). ArticleGoogle Scholar
- Fan, Y.-T., Decety, J., Yang, C.-Y., Liu, J.-L. & Cheng, Y. Unbroken mirror neurons in autism spectrum disorders. J. Child Psychol. Psychiatry51, 981–988 (2010). ArticlePubMedGoogle Scholar
- Southgate, V. & Hamilton, A. F. Unbroken mirrors: challenging a theory of autism. Trends Cogn. Sci.12, 225–229 (2008). ArticlePubMedGoogle Scholar
- Bernier, R., Aaronson, B. & McPartland, J. The role of imitation in the observed heterogeneity in EEG mu rhythm in autism and typical development. Brain Cogn.82, 69–75 (2013). ArticlePubMedGoogle Scholar
- Raymaekers, R., Wiersema, J. R. & Roeyers, H. EEG study of the mirror neuron system in children with high functioning autism. Brain Res.1304, 113–121 (2009). ArticleCASPubMedGoogle Scholar
- Dumas, G., Soussignan, R., Hugueville, L., Martinerie, J. & Nadel, J. Revisiting mu suppression in autism spectrum disorder. Brain Res.1585, 108–119 (2014). This paper replicates the mu suppression deficits in autism during action observation but questions, through high-density spectral analyses and source reconstruction, its previously drawn relation to the mirror neuron system.ArticleCASPubMedGoogle Scholar
- Marco, E. J., Hinkley, L. B. N., Hill, S. S. & Nagarajan, S. S. Sensory processing in autism: a review of neurophysiologic findings. Pediatr. Res.69, 48R–54R (2011). ArticlePubMedPubMed CentralGoogle Scholar
- Schwartz, S., Shinn-Cunningham, B. & Tager-Flusberg, H. Meta-analysis and systematic review of the literature characterizing auditory mismatch negativity in individuals with autism. Neurosci. Biobehav. Rev.87, 106–117 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Kang, E. et al. Atypicality of the N170 event-related potential in autism spectrum disorder: a meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging3, 657–666 (2018). ArticlePubMedGoogle Scholar
- Bonnet-Brilhault, F. et al. GABA/glutamate synaptic pathways targeted by integrative genomic and electrophysiological explorations distinguish autism from intellectual disability. Mol. Psychiatry21, 411–418 (2016). ArticleCASPubMedGoogle Scholar
- Schilbach, L. Towards a second-person neuropsychiatry. Phil. Trans. R. Soc. B371, 20150081 (2016). This review supports that psychiatric disorders are more commonly characterized by impairments of social interaction rather than social observation, and advocates for an interactive turn in neuropsychiatry. ArticlePubMedPubMed CentralGoogle Scholar
- Barraza, P. et al. Implementing EEG hyperscanning setups. MethodsX6, 428–436 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Dumas, G., de Guzman, G. C., Tognoli, E. & Kelso, J. A. The human dynamic clamp as a paradigm for social interaction. Proc. Natl Acad. Sci. USA111, E3726–E3734 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Jones, E. J. H. et al. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: a longitudinal prospective study of infants at high familial risk. J. Neurodev. Disord.8, 7 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ciarrusta, J. et al. Social brain functional maturation in newborn infants with and without a family history of autism spectrum disorder. JAMA Netw. Open2, e191868 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Levin, A. R., Varcin, K. J., O’Leary, H. M., Tager-Flusberg, H. & Nelson, C. A. EEG power at 3 months in infants at high familial risk for autism. J. Neurodev. Disord.9, 34 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Kolesnik, A. et al. Increased cortical reactivity to repeated tones at 8 months in infants with later ASD. Transl. Psychiatry9, 46 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Rippon, G., Brock, J., Brown, C. & Boucher, J. Disordered connectivity in the autistic brain: challenges for the ‘new psychophysiology’. Int. J. Psychophysiol.63, 164–172 (2007). ArticlePubMedGoogle Scholar
- Rosenberg, A., Patterson, J. S. & Angelaki, D. E. A computational perspective on autism. Proc. Natl Acad. Sci. USA112, 9158–9165 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Masuda, F. et al. Motor cortex excitability and inhibitory imbalance in autism spectrum disorder assessed with transcranial magnetic stimulation: a systematic review. Transl. Psychiatry9, 110 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- O’Reilly, C., Lewis, J. D. & Elsabbagh, M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLOS ONE12, e0175870 (2017). ArticlePubMedPubMed CentralCASGoogle Scholar
- Khan, S. et al. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain138, 1394–1409 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Chen, H., Nomi, J. S., Uddin, L. Q., Duan, X. & Chen, H. Intrinsic functional connectivity variance and state-specific under-connectivity in autism. Hum. Brain Mapp.38, 5740–5755 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Catarino, A., Churches, O., Baron-Cohen, S., Andrade, A. & Ring, H. Atypical EEG complexity in autism spectrum conditions: a multiscale entropy analysis. Clin. Neurophysiol.122, 2375–2383 (2011). ArticlePubMedGoogle Scholar
- Engemann, D. A. et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain141, 3179–3192 (2018). ArticlePubMedGoogle Scholar
- Open Science Collaboration. Psychology. Estimating the reproducibility of psychological science. Science349, aac4716 (2015). ArticleCASGoogle Scholar
- Lord, C. et al. Autism diagnostic observation schedule: ADOS-2 (Western Psychological Services, 2012).
- Regier, D. A. et al. DSM-5 field trials in the United States and Canada, part II: test-retest reliability of selected categorical diagnoses. Am. J. Psychiatry170, 59–70 (2013). ArticlePubMedGoogle Scholar
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edn (American Psychiatric Association, 2013).
- World Health Organization. International classification of diseases for mortality and morbidity statistics (11th Revision). https://icd.who.int/browse11/l-m/en (WHO, 2018).
- Constantino, J. N. & Charman, T. Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurol.15, 279–291 (2016). ArticlePubMedGoogle Scholar
- Lord, C. A multisite study of the clinical diagnosis of different autism spectrum disorders. Arch. Gen. Psychiatry69, 306–313 (2012). ArticlePubMedGoogle Scholar
- Miller, J. N. & Ozonoff, S. The external validity of Asperger disorder: lack of evidence from the domain of neuropsychology. J. Abnorm. Psychol.109, 227–238 (2000). ArticleCASPubMedGoogle Scholar
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edn (American Psychiatric Association, 1994).
- Green, D., Chandler, S., Charman, T., Simonoff, E. & Baird, G. Brief report: DSM-5 sensory behaviours in children with and without an autism spectrum disorder. J. Autism Dev. Disord.46, 3597–3606 (2016). ArticlePubMedGoogle Scholar
- Ozonoff, S. et al. Diagnosis of autism spectrum disorder after age 5 in children evaluated longitudinally since infancy. J. Am. Acad. Child Adolesc. Psychiatry57, 849–857.e2 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Russell, G., Steer, C. & Golding, J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc. Psychiatry Psychiatr. Epidemiol.46, 1283–1293 (2011). ArticlePubMedGoogle Scholar
- Charman, T. & Gotham, K. Measurement issues: screening and diagnostic instruments for autism spectrum disorders—lessons from research and practice. Child Adolesc. Ment. Health18, 52–63 (2013). ArticlePubMedGoogle Scholar
- Ashwood, K. L., Buitelaar, J., Murphy, D., Spooren, W. & Charman, T. European clinical network: autism spectrum disorder assessments and patient characterisation. Eur. Child Adolesc. Psychiatry24, 985–995 (2015). ArticlePubMedGoogle Scholar
- Rutter, M., LeCouteur, A. & Lord, C. Autism Diagnostic Interview-Revised (ADI-R). (Western Psychological Services, 2003).
- Durkin, M. S. et al. Autism screening and diagnosis in low resource settings: challenges and opportunities to enhance research and services worldwide. Autism Res.8, 473–476 (2015). This position paper highlights the challenges to translating knowledge on better awareness, understanding, identification and diagnosis (and then treatments) from the past two decades of clinical research in high-income countries into low-income and middle-income countries.ArticlePubMedPubMed CentralGoogle Scholar
- Baird, G. et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet368, 210–215 (2006). ArticlePubMedGoogle Scholar
- Luyster, R. et al. The autism diagnostic observation schedule — toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J. Autism Dev. Disord.39, 1305–1320 (2009). ArticlePubMedPubMed CentralGoogle Scholar
- de Vries, P. J. Thinking globally to meet local needs: autism spectrum disorders in Africa and other low-resource environments. Curr. Opin. Neurol.29, 130–136 (2016). ArticlePubMedCASGoogle Scholar
- Georgiades, S., Bishop, S. L. & Frazier, T. Editorial perspective: longitudinal research in autism—introducing the concept of ‘chronogeneity’. J. Child Psychol. Psychiatry58, 634–636 (2017). ArticlePubMedGoogle Scholar
- Fountain, C., Winter, A. S. & Bearman, P. S. Six developmental trajectories characterize children with autism. Pediatrics129, e1112–e1120 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Kim, S. H. et al. Variability in autism symptom trajectories using repeated observations from 14 to 36 months of age. J. Am. Acad. Child Adolesc. Psychiatry57, 837–848.e2 (2018). ArticlePubMedPubMed CentralGoogle Scholar
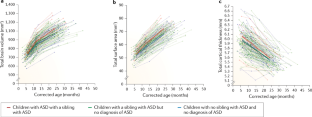
- Bussu, G. et al. Latent trajectories of adaptive behaviour in infants at high and low familial risk for autism spectrum disorder. Mol. Autism10, 13 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Zerbi, V. et al. Dysfunctional autism risk genes cause circuit-specific connectivity deficits with distinct developmental trajectories. Cereb. Cortex28, 2495–2506 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Fein, D. et al. Optimal outcome in individuals with a history of autism. J. Child Psychol. Psychiatry54, 195–205 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Anderson, D. K., Liang, J. W. & Lord, C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry55, 485–494 (2014). ArticlePubMedGoogle Scholar
- Chlebowski, C., Robins, D. L., Barton, M. L. & Fein, D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics131, e1121–e1127 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Stenberg, N. et al. Identifying children with autism spectrum disorder at 18 months in a general population sample. Paediatr. Perinat. Epidemiol.28, 255–262 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Pierce, K., Courchesne, E. & Bacon, E. To screen or not to screen universally for autism is not the question: why the task force got it wrong. J. Pediatr.176, 182–194 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Siu, A. L. et al. Screening for autism spectrum disorder in young children: US Preventive Services Task Force recommendation statement. JAMA315, 691–696 (2016). ArticleCASPubMedGoogle Scholar
- Øien, R. A. et al. Clinical features of children with autism who passed 18-month screening. Pediatrics141, e20173596 (2018). ArticlePubMedGoogle Scholar
- Sánchez-García, A. B., Galindo-Villardón, P., Nieto-Librero, A. B., Martín-Rodero, H. & Robins, D. L. Toddler screening for autism spectrum disorder: a meta-analysis of diagnostic accuracy. J. Autism Dev. Disord.49, 1837–1852 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Marlow, M., Servili, C. & Tomlinson, M. A review of screening tools for the identification of autism spectrum disorders and developmental delay in infants and young children: recommendations for use in low- and middle-income countries. Autism Res.12, 176–199 (2019). ArticlePubMedGoogle Scholar
- Raza, S. et al. Brief report: evaluation of the short quantitative checklist for autism in toddlers (Q-CHAT-10) as a brief screen for autism spectrum disorder in a high-risk sibling cohort. J. Autism Dev. Disord.49, 2210–2218 (2019). ArticlePubMedGoogle Scholar
- Charman, T. et al. Testing two screening instruments for autism spectrum disorder in UK community child health services. Dev. Med. Child Neurol.58, 369–375 (2016). ArticlePubMedGoogle Scholar
- Brett, D., Warnell, F., McConachie, H. & Parr, J. R. Factors affecting age at ASD diagnosis in UK: no evidence that diagnosis age has decreased between 2004 and 2014. J. Autism Dev. Disord.46, 1974–1984 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Zuckerman, K. E., Lindly, O. J. & Sinche, B. K. Parental concerns, provider response, and timeliness of autism spectrum disorder diagnosis. J. Pediatr.166, 1431–1439.e1 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Boterberg, S., Charman, T., Marschik, P. B., Bölte, S. & Roeyers, H. Regression in autism spectrum disorder: a critical overview of retrospective findings and recommendations for future research. Neurosci. Biobehav. Rev.102, 24–55 (2019). ArticlePubMedGoogle Scholar
- Pearson, N., Charman, T., Happé, F., Bolton, P. F. & McEwen, F. S. Regression in autism spectrum disorder: reconciling findings from retrospective and prospective research. Autism Res.11, 1602–1620 (2018). ArticlePubMedGoogle Scholar
- Ozonoff, S. & Iosif, A.-M. Changing conceptualizations of regression: what prospective studies reveal about the onset of autism spectrum disorder. Neurosci. Biobehav. Rev.100, 296–304 (2019). Despite its potential importance as a biological marker and/or subgroup of ASD, developmental regression has remained very poorly understood. This paper outlines recent data and reconceptualization about patterns of onset (and loss) that chime with a more contemporaneous understanding of ASD as a heterogeneous condition in terms of its manifestation both within and across individuals. ArticlePubMedPubMed CentralGoogle Scholar
- Brugha, T. S. et al. Validating two survey methods for identifying cases of autism spectrum disorder among adults in the community. Psychol. Med.42, 647–656 (2012). ArticleCASPubMedGoogle Scholar
- Brugha, T. S. The Psychiatry of Adult Autism and Asperger Syndrome: a Practical Guide (Oxford Univ. Press, 2018).
- Epstein, J., Johnson, D. E. & Conners, C. K. Conners Adult ADHD Diagnostic Interview for DSM-IV (CAADID) (MHS, 2001).
- Lai, M.-C. et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry6, 819–829 (2019). ArticlePubMedGoogle Scholar
- Havdahl, A. & Bishop, S. Heterogeneity in prevalence of co-occurring psychiatric conditions in autism. Lancet Psychiatry6, 794–795 (2019). ArticlePubMedGoogle Scholar
- Croen, L. A. et al. The health status of adults on the autism spectrum. Autism19, 814–823 (2015). ArticlePubMedGoogle Scholar
- Mannion, A., Leader, G. & Healy, O. An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with autism spectrum disorder. Res. Autism Spectr. Disord.7, 35–42 (2013). ArticleGoogle Scholar
- Soke, G. N., Maenner, M. J., Christensen, D., Kurzius-Spencer, M. & Schieve, L. A. Prevalence of co-occurring medical and behavioral conditions/symptoms among 4- and 8-year-old children with autism spectrum disorder in selected areas of the United States in 2010. J. Autism Dev. Disord.48, 2663–2676 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Chandler, S. et al. Emotional and behavioural problems in young children with autism spectrum disorder. Dev. Med. Child Neurol.58, 202–208 (2016). ArticlePubMedGoogle Scholar
- Pezzimenti, F., Han, G. T., Vasa, R. A. & Gotham, K. Depression in youth with autism spectrum disorder. Child Adolesc. Psychiatr. Clin. N. Am.28, 397–409 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- Hwang, Y. I. J., Srasuebkul, P., Foley, K. R., Arnold, S. & Trollor, J. N. Mortality and cause of death of Australians on the autism spectrum. Autism Res.12, 806–815 (2019). ArticleCASPubMedGoogle Scholar
- Hirvikoski, T. et al. Premature mortality in autism spectrum disorder. Br. J. Psychiatry208, 232–238 (2016). ArticlePubMedGoogle Scholar
- Havdahl, K. A. et al. Multidimensional influences on autism symptom measures: implications for use in etiological research. J. Am. Acad. Child Adolesc. Psychiatry55, 1054–1063.e3 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Nicolaidis, C. et al. Comparison of healthcare experiences in autistic and non-autistic adults: a cross-sectional online survey facilitated by an academic-community partnership. J. Gen. Intern. Med.28, 761–769 (2013). ArticlePubMedGoogle Scholar
- Schreibman, L. et al. Naturalistic developmental behavioral interventions: empirically validated treatments for autism spectrum disorder. J. Autism Dev. Disord.45, 2411–2428 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Tomlinson, M. et al. Setting global research priorities for developmental disabilities, including intellectual disabilities and autism: setting research priorities for developmental disabilities. J. Intellect. Disabil. Res.58, 1121–1130 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Rahman, A. et al. Effectiveness of the parent-mediated intervention for children with autism spectrum disorder in South Asia in India and Pakistan (PASS): a randomised controlled trial. Lancet Psychiatry3, 128–136 (2016). ArticlePubMedGoogle Scholar
- Lovaas, O. I. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J. Consult. Clin. Psychol.55, 3–9 (1987). ArticleCASPubMedGoogle Scholar
- Nevill, R. E., Lecavalier, L. & Stratis, E. A. Meta-analysis of parent-mediated interventions for young children with autism spectrum disorder. Autism22, 84–98 (2018). ArticlePubMedGoogle Scholar
- Kasari, C. et al. Randomized controlled trial of parental responsiveness intervention for toddlers at high risk for autism. Infant Behav. Dev.37, 711–721 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Shire, S. Y. et al. Hybrid implementation model of community-partnered early intervention for toddlers with autism: a randomized trial. J. Child Psychol. Psychiatry58, 612–622 (2017). ArticlePubMedGoogle Scholar
- Siller, M., Hutman, T. & Sigman, M. A parent-mediated intervention to increase responsive parental behaviors and child communication in children with ASD: a randomized clinical trial. J. Autism Dev. Disord.43, 540–555 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Rogers, S. J. et al. Effects of a brief early start denver model (ESDM)-based parent intervention on toddlers at risk for autism spectrum disorders: a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry51, 1052–1065 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Green, J. et al. Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet375, 2152–2160 (2010). ArticlePubMedPubMed CentralGoogle Scholar
- Pickles, A. et al. Parent-mediated social communication therapy for young children with autism (PACT): long-term follow-up of a randomised controlled trial. Lancet388, 2501–2509 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Dawson, G. et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics125, e17–e23 (2010). ArticlePubMedGoogle Scholar
- Charman, T. Editorial: trials and tribulations in early autism intervention research. J. Am. Acad. Child Adolesc. Psychiatry58, 846–848 (2019). ArticlePubMedGoogle Scholar
- Rogers, S. J. et al. A multisite randomized controlled two-phase trial of the early start denver model compared to treatment as usual. J. Am. Acad. Child Adolesc. Psychiatry58, 853–865 (2019). ArticlePubMedGoogle Scholar
- Dawson, G. et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry51, 1150–1159 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Myers, S. M., Johnson, C. P. & The Council on Children With Disabilities. Management of children with autism spectrum disorders. Pediatrics120, 1162–1182 (2007). ArticlePubMedGoogle Scholar
- Laugeson, E. A., Frankel, F., Gantman, A., Dillon, A. R. & Mogil, C. Evidence-based social skills training for adolescents with autism spectrum disorders: the UCLA PEERS program. J. Autism Dev. Disord.42, 1025–1036 (2012). ArticlePubMedGoogle Scholar
- Reichow, B., Servili, C., Yasamy, M. T., Barbui, C. & Saxena, S. Non-specialist psychosocial interventions for children and adolescents with intellectual disability or lower-functioning autism spectrum disorders: a systematic review. PLOS Med.10, e1001572 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Brignell, A. et al. Communication interventions for autism spectrum disorder in minimally verbal children. Cochrane Database Syst. Rev.11, CD012324 (2018). PubMedGoogle Scholar
- Tarver, J. et al. Child and parent outcomes following parent interventions for child emotional and behavioral problems in autism spectrum disorders: a systematic review and meta-analysis. Autism23, 1630–1644 (2019). ArticlePubMedGoogle Scholar
- Keefer, A. et al. Exploring relationships between negative cognitions and anxiety symptoms in youth with autism spectrum disorder. Behav. Ther.49, 730–740 (2018). ArticlePubMedGoogle Scholar
- Bearss, K. et al. Effect of parent training vs parent education on behavioral problems in children with autism spectrum disorder: a randomized clinical trial. JAMA313, 1524–1533 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Da Paz, N. S. & Wallander, J. L. Interventions that target improvements in mental health for parents of children with autism spectrum disorders: a narrative review. Clin. Psychol. Rev.51, 1–14 (2017). ArticlePubMedGoogle Scholar
- Kasari, C. et al. Children with autism spectrum disorder and social skills groups at school: a randomized trial comparing intervention approach and peer composition. J. Child Psychol. Psychiatry57, 171–179 (2016). ArticlePubMedGoogle Scholar
- Marshall, D. et al. Social stories in mainstream schools for children with autism spectrum disorder: a feasibility randomised controlled trial. BMJ Open6, e011748 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Taylor, J. L. et al. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics130, 531–538 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Pallathra, A. A., Cordero, L., Wong, K. & Brodkin, E. S. Psychosocial interventions targeting social functioning in adults on the autism spectrum: a literature review. Curr. Psychiatry Rep.21, 5 (2019). ArticlePubMedPubMed CentralGoogle Scholar
- White, S. W. et al. Psychosocial treatments targeting anxiety and depression in adolescents and adults on the autism spectrum: review of the latest research and recommended future directions. Curr. Psychiatry Rep.20, 82 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Shattuck, P. T., Wagner, M., Narendorf, S., Sterzing, P. & Hensley, M. Post-high school service use among young adults with an autism spectrum disorder. Arch. Pediatr. Adolesc. Med.165, 141–146 (2011). ArticlePubMedPubMed CentralGoogle Scholar
- Wehman, P. et al. Effects of an employer-based intervention on employment outcomes for youth with significant support needs due to autism. Autism21, 276–290 (2017). ArticlePubMedGoogle Scholar
- McCracken, J. T. et al. Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med.347, 314–321 (2002). ArticleCASPubMedGoogle Scholar
- Owen, R. et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics124, 1533–1540 (2009). ArticlePubMedGoogle Scholar
- McPheeters, M. L. et al. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics127, e1312–e1321 (2011). ArticlePubMedGoogle Scholar
- Anagnostou, E. et al. Metformin for treatment of overweight induced by atypical antipsychotic medication in young people with autism spectrum disorder: a randomized clinical trial. JAMA Psychiatry73, 928–937 (2016). ArticlePubMedGoogle Scholar
- Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry62, 1266–1274 (2005). ArticleGoogle Scholar
- Handen, B. L. et al. Atomoxetine, parent training, and their combination in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry54, 905–915 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Scahill, L. et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am. J. Psychiatry172, 1197–1206 (2015). ArticlePubMedGoogle Scholar
- Hollander, E. et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am. J. Psychiatry169, 292–299 (2012). ArticlePubMedGoogle Scholar
- King, B. H. et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch. Gen. Psychiatry66, 583–590 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Anagnostou, E. et al. Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res.1580, 188–198 (2014). ArticleCASPubMedGoogle Scholar
- Guastella, A. J. et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J. Child Psychol. Psychiatry56, 444–452 (2015). ArticlePubMedGoogle Scholar
- Parker, K. J. et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med.11, eaau7356 (2019). ArticleCASPubMedPubMed CentralGoogle Scholar
- Bolognani, F. et al. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med.11, eaat7838 (2019). ArticleCASPubMedGoogle Scholar
- Rubenstein, J. L. R. & Merzenich, M. M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav.2, 255–267 (2003). ArticleCASPubMedPubMed CentralGoogle Scholar
- Veenstra-VanderWeele, J. et al. Arbaclofen in children and adolescents with autism spectrum disorder: a randomized, controlled, phase 2 trial. Neuropsychopharmacology42, 1390–1398 (2017). ArticleCASPubMedGoogle Scholar
- Berry-Kravis, E. et al. Mavoglurant in fragile X syndrome: results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med.8, 321ra5 (2016). ArticlePubMedCASGoogle Scholar
- Krueger, D. A. et al. Everolimus for treatment of tuberous sclerosis complex-associated neuropsychiatric disorders. Ann. Clin. Transl. Neurol.4, 877–887 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Georgiades, S. & Kasari, C. Reframing optimal outcomes in autism. JAMA Pediatr.172, 716–717 (2018). ArticlePubMedGoogle Scholar
- Bishop-Fitzpatrick, L. et al. Characterizing objective quality of life and normative outcomes in adults with autism spectrum disorder: an exploratory latent class analysis. J. Autism Dev. Disord.46, 2707–2719 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol. Med.28, 551–558 (1998). ArticleGoogle Scholar
- Gotham, K. et al. Characterizing the daily life, needs, and priorities of adults with autism spectrum disorder from interactive autism network data. Autism19, 794–804 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Taylor, J. L. & Seltzer, M. M. Employment and post-secondary educational activities for young adults with autism spectrum disorders during the transition to adulthood. J. Autism Dev. Disord.41, 566–574 (2011). ArticlePubMedPubMed CentralGoogle Scholar
- Orsmond, G. I., Shattuck, P. T., Cooper, B. P., Sterzing, P. R. & Anderson, K. A. Social participation among young adults with an autism spectrum disorder. J. Autism Dev. Disord.43, 2710–2719 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Henninger, N. A. & Taylor, J. L. Outcomes in adults with autism spectrum disorders: a historical perspective. Autism17, 103–116 (2013). ArticlePubMedGoogle Scholar
- Howlin, P. & Moss, P. Adults with autism spectrum disorders. Can. J. Psychiatry57, 275–283 (2012). ArticlePubMedGoogle Scholar
- Farley, M. A. et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Res.2, 109–118 (2009). ArticlePubMedGoogle Scholar
- Taylor, J. L., Henninger, N. A. & Mailick, M. R. Longitudinal patterns of employment and postsecondary education for adults with autism and average-range IQ. Autism19, 785–793 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Lai, M.-C. et al. Quantifying and exploring camouflaging in men and women with autism. Autism21, 690–702 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- van Heijst, B. F. & Geurts, H. M. Quality of life in autism across the lifespan: a meta-analysis. Autism19, 158–167 (2015). ArticlePubMedGoogle Scholar
- Moss, P., Mandy, W. & Howlin, P. Child and adult factors related to quality of life in adults with autism. J. Autism Dev. Disord.47, 1830–1837 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Bishop-Fitzpatrick, L., Mazefsky, C. A. & Eack, S. M. The combined impact of social support and perceived stress on quality of life in adults with autism spectrum disorder and without intellectual disability. Autism22, 703–711 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Kamio, Y., Inada, N. & Koyama, T. A nationwide survey on quality of life and associated factors of adults with high-functioning autism spectrum disorders. Autism17, 15–26 (2013). ArticlePubMedGoogle Scholar
- Mason, D. et al. Predictors of quality of life for autistic adults. Autism Res.11, 1138–1147 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Autistica. Your questions shaping future autism research. https://www.autistica.org.uk/downloads/files/Autism-Top-10-Your-Priorities-for-Autism-Research.pdf (2016).
- Ontario Brain Institute. Community priorities for research on neurodevelopmental disorders. http://braininstitute.ca/img/JLA-NDD-Final-Report.pdf (2018).
- den Houting, J. Neurodiversity: an insider’s perspective. Autism23, 271–273 (2018). ArticleGoogle Scholar
- Szatmari, P. Risk and resilience in autism spectrum disorder: a missed translational opportunity? Dev. Med. Child Neurol.60, 225–229 (2018). ArticlePubMedGoogle Scholar
- Markowitz, L. A. et al. Development and psychometric evaluation of a psychosocial quality-of-life questionnaire for individuals with autism and related developmental disorders. Autism20, 832–844 (2016). ArticlePubMedGoogle Scholar
- Ryan, S. & Cole, K. R. From advocate to activist? Mapping the experiences of mothers of children on the autism spectrum. J. Appl. Res. Intellect. Disabil.22, 43–53 (2009). ArticleGoogle Scholar
- McCann, D., Bull, R. & Winzenberg, T. The daily patterns of time use for parents of children with complex needs: a systematic review. J. Child Health Care16, 26–52 (2012). ArticlePubMedGoogle Scholar
- Karst, J. S. & Van Hecke, A. V. Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation. Clin. Child Fam. Psychol. Rev.15, 247–277 (2012). ArticlePubMedGoogle Scholar
- Lounds, J., Seltzer, M. M., Greenberg, J. S. & Shattuck, P. T. Transition and change in adolescents and young adults with autism: longitudinal effects on maternal well-being. Am. J. Ment. Retard.112, 401–417 (2007). ArticlePubMedGoogle Scholar
- Burke, M. & Heller, T. Individual, parent and social-environmental correlates of caregiving experiences among parents of adults with autism spectrum disorder. J. Intellect. Disabil. Res.60, 401–411 (2016). ArticleCASPubMedGoogle Scholar
- Kim, S. H., Bal, V. H. & Lord, C. Longitudinal follow-up of academic achievement in children with autism from age 2 to 18. J. Child Psychol. Psychiatry59, 258–267 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Lord, C., Bishop, S. & Anderson, D. Developmental trajectories as autism phenotypes. Am. J. Med. Genet. C Semin. Med. Genet.169, 198–208 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Global Research on Developmental Disabilities Collaborators. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancel Glob. Health6, e1100–e1121 (2018).
- Kahn, R. S. et al. Schizophrenia. Nat. Rev. Dis. Primers1, 15067 (2015). ArticlePubMedGoogle Scholar
- Patel, V. et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet387, 1672–1685 (2016). ArticlePubMedGoogle Scholar
- Franz, L., Chambers, N., von Isenburg, M. & de Vries, P. J. Autism spectrum disorder in sub-Saharan Africa: a comprehensive scoping review. Autism Res.10, 723–749 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- World Health Organization. Training parents to transform children’s lives. https://www.who.int/mental_health/maternal-child/PST/en/ (WHO, 2019).
- Naslund, J. A. et al. Digital innovations for global mental health: opportunities for data science, task sharing, and early intervention. Curr. Treat. Options Psychiatryhttps://doi.org/10.1007/s40501-019-00186-8 (2019).
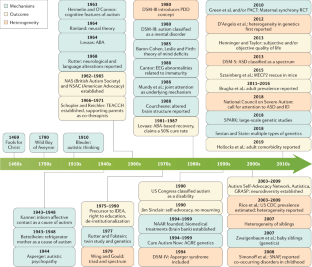
- Sadowsky, J., Donvan, J. & Zucker, C. In a different key: the story of autism. J. Hist. Behav. Sci.54, 66–67 (2018). This paper presents a different, broad overview of the changes in perspective about autism and ASD over the years. ArticleGoogle Scholar
- Rutter, M., Greenfeld, D. & Lockyer, L. A five to fifteen year follow-up study of infantile psychosis. II. Social and behavioural outcome. Br. J. Psychiatry113, 1183–1199 (1967). ArticleCASPubMedGoogle Scholar
- Hermelin, B. & O’Connor, N. Psychological Experiments with Autistic Children (Pergamon Press, 1970).
- Rimland, B. Infantile Autism: the Syndrome and its Implications for a Neural Theory of Behaviour (Meredith Publishing Company, 1964).
- Frith, U. Studies in pattern detection in normal and autistic children: I. Immediate recall of auditory sequences. J. Abnorm. Psychol.76, 413–420 (1970). ArticleCASPubMedGoogle Scholar
- Folstein, S. & Rutter, M. in Autism (eds. Rutter M. & Schopler E.) 219–241 (Springer, 1978).
- Mundy, P., Sigman, M. & Kasari, C. A longitudinal study of joint attention and language development in autistic children. J. Autism Dev. Disord.20, 115–128 (1990). ArticleCASPubMedGoogle Scholar
- Schopler, E. & Reichler, R. J. Parents as cotherapists in the treatment of psychotic children. J. Autism Child. Schizophr.1, 87–102 (1971). ArticleCASPubMedGoogle Scholar
- Sinclair, J. Don’t mourn for us. Autism Network Internationalhttp://www.autreat.com/dont_mourn.html (1993).
- Wing, L. & Gould, J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord.9, 11–29 (1979). ArticleCASPubMedGoogle Scholar
- Chawner, S. et al. A genetic first approach to dissecting the heterogeneity of autism: phenotypic comparison of autism risk copy number variants. Eur. Neuropsychopharmacol.29 (Suppl. 3), S783–S784 (2019). ArticleGoogle Scholar
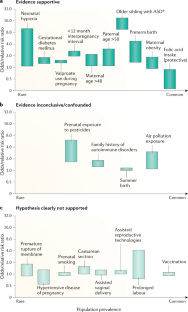
- Modabbernia, A., Mollon, J., Boffetta, P. & Reichenberg, A. Impaired gas exchange at birth and risk of intellectual disability and autism: a meta-analysis. J. Autism Dev. Disord.46, 1847–1859 (2016). ArticlePubMedGoogle Scholar
- Christensen, J. et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA309, 1696–1703 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Xie, F., Peltier, M. & Getahun, D. Is the risk of autism in younger siblings of affected children moderated by sex, race/ethnicity, or gestational age? J. Dev. Behav. Pediatr.37, 603–609 (2016). ArticlePubMedGoogle Scholar
- Guy, A. et al. Infants born late/moderately preterm are at increased risk for a positive autism screen at 2 years of age. J. Pediatr.166, 269–275.e3 (2015). ArticlePubMedGoogle Scholar
- Schendel, D. & Bhasin, T. K. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics121, 1155–1164 (2008). ArticlePubMedGoogle Scholar
- Windham, G. C. et al. Maternal pre-pregnancy body mass index and gestational weight gain in relation to autism spectrum disorder and other developmental disorders in offspring. Autism Res.12, 316–327 (2019). ArticlePubMedGoogle Scholar
- Schmidt, R. J. et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr.96, 80–89 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Conde-Agudelo, A., Rosas-Bermudez, A. & Norton, M. H. Birth spacing and risk of autism and other neurodevelopmental disabilities: a systematic review. Pediatrics137, e20153482 (2016). ArticlePubMedGoogle Scholar
- Lyall, K. et al. The changing epidemiology of autism spectrum disorders. Annu. Rev. Public Health38, 81–102 (2017). ArticlePubMedGoogle Scholar
- Cheslack-Postava, K., Liu, K. & Bearman, P. S. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics127, 246–253 (2011). ArticlePubMedPubMed CentralGoogle Scholar
- Conti, E., Mazzotti, S., Calderoni, S., Saviozzi, I. & Guzzetta, A. Are children born after assisted reproductive technology at increased risk of autism spectrum disorders? A systematic review. Hum. Reprod.28, 3316–3327 (2013). ArticleCASPubMedGoogle Scholar
- Lehti, V. et al. Autism spectrum disorders in IVF children: a national case-control study in Finland. Hum. Reprod.28, 812–818 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Rossignol, D. A., Genuis, S. J. & Frye, R. E. Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psychiatry4, e360 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Curran, E. A. et al. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J. Child Psychol. Psychiatry56, 500–508 (2015). ArticlePubMedGoogle Scholar
- Chandler, S., Howlin, P., Simonoff, E., Kennedy, J. & Baird, G. Comparison of parental estimate of developmental age with measured IQ in children with neurodevelopmental disorders. Child Care Health Dev.42, 486–493 (2016). ArticleCASPubMedGoogle Scholar
- Charman, T. et al. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP). Psychol. Med.41, 619–627 (2011). ArticleCASPubMedGoogle Scholar
- Sparrow, S. S., Cicchetti, D. & Balla, D. A. Vineland Adaptive Behavior Scales, 2nd Edn. https://doi.org/10.1037/t15164-000 (AGS, 2005).
- Jones, R. M., Pickles, A. & Lord, C. Evaluating the quality of peer interactions in children and adolescents with autism with the Penn Interactive Peer Play Scale (PIPPS). Mol. Autism8, 28 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Lord, C., Elsabbagh, M., Baird, G. & Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet392, 508–520 (2018). ArticlePubMedPubMed CentralGoogle Scholar
- Duncan, A. W. & Bishop, S. L. Understanding the gap between cognitive abilities and daily living skills in adolescents with autism spectrum disorders with average intelligence. Autism19, 64–72 (2015). ArticlePubMedGoogle Scholar
Acknowledgements
The authors thank J. McCauley, S. Gaspar, K. Byrne and A. Holbrook from UCLA for help with manuscript preparation. S. Tromans is thanked for his updated review of the epidemiology literature. We recognize the many investigators who contributed research that we cannot cite due to space limitations. C.L. is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHHD; R01 HD081199), the National Institute of Mental Health (NIMH; R01MH081873-01A1) and the Simons Foundation. T.S.B. is supported by grants from the Health and Social Care Information Centre, Leeds, and the National Institute for Health Research (NIHR HTA; grant ref. NIHR127337). T.C. is supported by grants from Innovative Medicines Initiative 2 (no. 777394), the Medical Research Council (MRC; grants MR/K021389/1) and the NIHR (grant 13/119/18). J.C. is funded by Autistica. G.D. is supported by the Institut Pasteur. T.F. is supported by the Autism Speaks Foundation. E.J.H.J. is supported by grants from the Economic and Social Research Council (ESRC; ES/R009368/1), the Innovative Medicines Initiative 2 (no. 777394), the MRC (MR/K021389/1) and the Simons Foundation (609081). R.M.J. acknowledges the Mortimer D. Sackler Family and the NIMH (R01MH114999). J.L.T. is supported by grants from the FAR fund and the NIMH (R34 MH104428, R03 MH 112783 and R01 MH116058). A.P. is partially supported by the Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London and the NIHR (NF-SI-0617-10120). M.W.S. is supported by the National Institutes of Health (NIH; MH106934, MH109901, MH110928, MH116487 MH102342, MH111662, MH105575 and MH115747), the Overlook International Foundation and the Simons Foundation. J.V.-V. is supported by the NIH (MH016434 and MH094604), the Simons Foundation and the New York State Psychiatric Institute. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Author information
Authors and Affiliations
- Departments of Psychiatry and School of Education, University of California, Los Angeles, Los Angeles, CA, USA Catherine Lord
- Department of Health Sciences, University of Leicester, Leicester, UK Traolach S. Brugha
- Institute of Psychiatry, Psychology & Neuroscience, King’s College London, London, UK Tony Charman & Andrew Pickles
- Autistica, London, UK James Cusack
- Institut Pasteur, UMR3571 CNRS, Université de Paris, Paris, France Guillaume Dumas
- Autism Speaks, New York, NY, USA Thomas Frazier
- Centre for Brain & Cognitive Development, University of London, London, UK Emily J. H. Jones
- The Sackler Institute for Developmental Psychobiology, New York, NY, USA Rebecca M. Jones
- The Center for Autism and the Developing Brain, White Plains, NY, USA Rebecca M. Jones
- Department of Psychiatry, Langley Porter Psychiatric Institute and Weill Institute for Neurosciences, University of California, San Francisco, CA, USA Matthew W. State
- Department of Pediatrics and Vanderbilt Kennedy Center, Vanderbilt University Medical Center, Nashville, TN, USA Julie Lounds Taylor
- Department of Psychiatry, Columbia University, New York, NY, USA Jeremy Veenstra-VanderWeele
- Catherine Lord











